Mixing Technology for Pharmaceutical Products
Agitators and Vacuum Processing Units
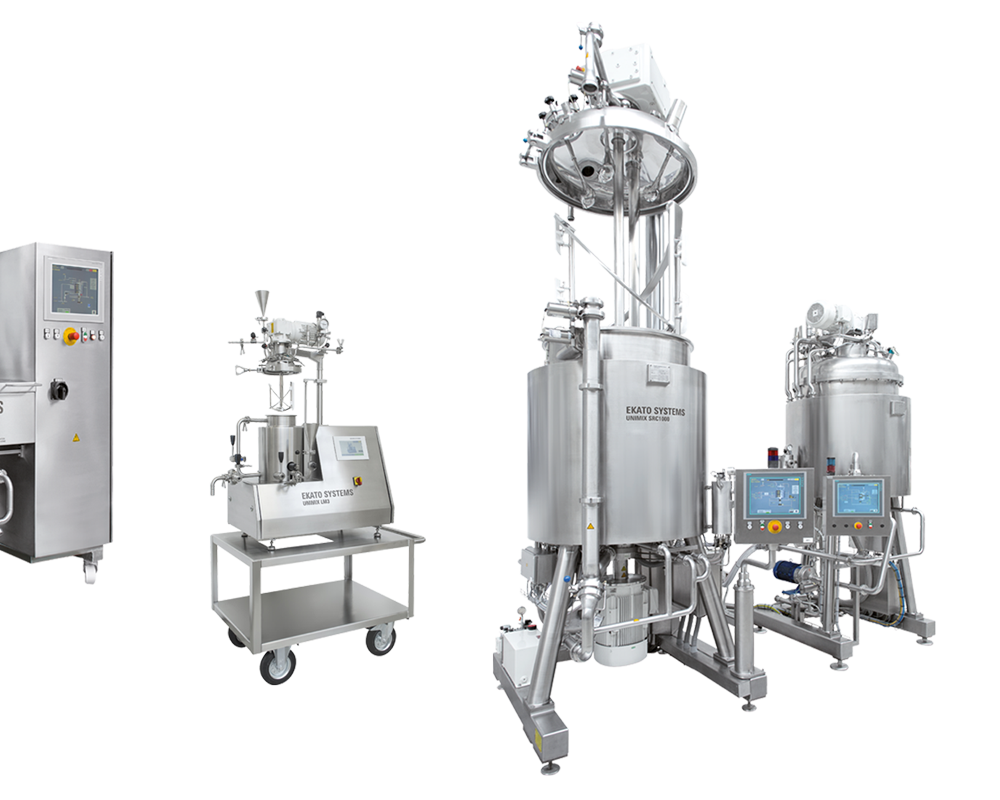
EKATO SYSTEMS for the Production of Ointments, Cremes, and Gels

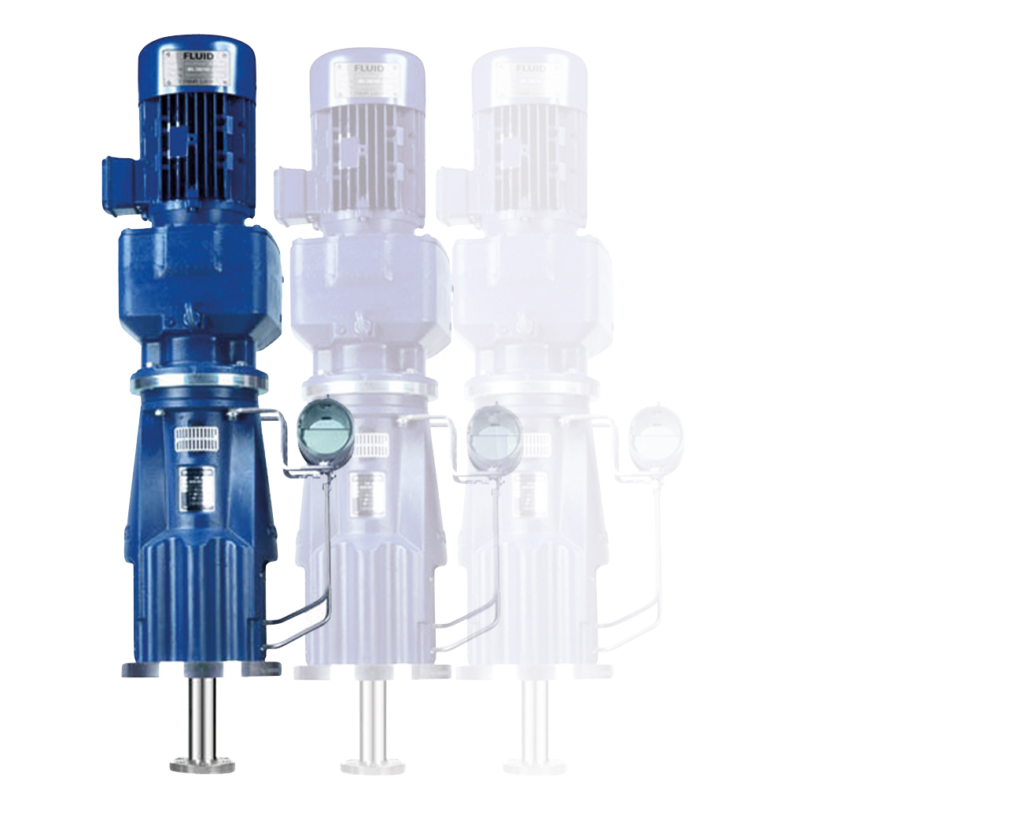
In addition to the challenging tasks of active pharmaceutical ingredient synthesis production, the EKATO GROUP can also cover the area of industrial agitators in the pharmaceutical industry through the mixing and dispersing technology of FLUID Misch- und Dispergiertechnik GmbH. Mixing tasks can be suspending solids in a liquid phase, heating and cooling or mixing liquids. These mixing tasks are often part of the starting materials or storage of intermediate and final products.
Scale-up capability of the technology is crucial. To achieve this, algorithms are applied that have been specifically developed and verified many times. The systems and equipment are subjected to an established certification process to ensure top product quality.
Typical Project Process Flow
- Concept phase
- Design phase
- Execution- and manufacturing phases
- Test phase
We support by our consulting competencies in all project phases beginning from an URS to a functional specification, execution specification, production and installation of the plant, including the necessary qualification, for example
- Design Qualification (DQ)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Support during Performance Qualification (PQ)
A crucial point is an early involvement and integration to the customers projects process to deliver the right package fitting customers’ needs.